WaveNowXV Electrochemical Workstation
The WaveNowXV electrochemical workstation is a capable, yet cost-effective, potentiostat and galvanostat system. For routine electrochemical measurements like cyclic voltammetry, chronoamperometry, square wave voltammetry, and many more DC techniques, the WaveNowXV is a great option for those looking for a small, simple, portable, and reliable electrochemical workstation. So small as to easily pass through a glovebox antechamber, low cost for educational electrochemistry, yet just as capable as any other basic potentiostat on the market. Customers often use this system for routine electrochemistry where impedance (EIS) is not required, which is how we are able to offer it for such a low price.
This product can only be purchased in bundles. View these related bundles in the tab below.
Customers must be logged into their account to view prices. Not all regions provide pricing online. If you do not see prices, you can obtain them from the designated sales channel in your region.
- Gupta, P.; Moore, C.E.; Turro, C. Photo- and Electrocatalytic Hydrogen Evolution by Heteroleptic Dirhodium(II,II) Complexes: Role of the Bridging and Diimine Ligands. J. Am. Chem. Soc. 2024, 146, 27161-27172.
- Esquivel-Castro, T.A.; Ceballos, J.; Torres-Zanoni, E.; Padmasree, K.P.; Valadez-Renteria, E.; Aldana-Sanchez, I.; Quevedo-Lopez, M.; Rodriguez-Gonzalez, V.; Oliva, J. Solar photocatalytic degradation of methylene blue dye and 4-CP herbicide by using a biodegradable fiber support decorated with Ce0.9Bi0.1O2 porous nanoparticles. Inorganic Chemistry Communications 2024, 168, 112946.
- Hossain, M. Oxidovanadium(V) complexes with tridentate hydrazone ligands as oxygen atom transfer catalysts. Polyhedron 2024, 258, 117020.
- Cole, H.D.; Vali, A.; Roque, J.A.I.; Shi, G.; Talgatov, A.; Kaur, G.; Francés-Monerris, A.; Alberto, M.E.; Cameron, C.G.; McFarland, S.A. Ru(II) Oligothienyl Complexes with Fluorinated Ligands: Photophysical, Electrochemical, and Photobiological Properties. Inorg. Chem. 2024, 63, 9735-9752.
- Eisnor, M.; Biton, M.; Granados, P.; Reader, H.; Brosseau, C. Coupling Multidimensional Chromatography with Plasmonic Sensing: An Exploration of Electrochemical SERS as a Detection Modality for 2D-LC. Can. J. Chem. 2024.
- Jayworth, J.A.; Decavoli, C.; Capobianco, M.D.; Menzel, J.P.; Adler, S.R.; Kocoj, C.A.; Freeze, J.G.; Crabtree, R.H.; Guo, P.; Batista, V.S.; Brudvig, G.W. BODIPY Chemisorbed on SnO2 and TiO2 Surfaces for Photoelectrochemical Applications. ACS Appl. Mater. Interfaces 2024, 16, 14841-14851.
- Durbin, M.M.; Balzer, A.H.; Reynolds, J.R.; Ratcliff, E.L.; Stingelin, N.; Österholm, A.M. Role of Side-Chain Free Volume on the Electrochemical Behavior of Poly(propylenedioxythiophenes). Chem. Mater. 2024, 36, 2634-2641.
- De Keersmaecker, M.; Garrett, B.S.; Shen, D.E.; Jones, A.L.; Österholm, A.M.; Mirotznik, M.; Reynolds, J.R. Conducting Polymer Switches Permit the Development of a Frequency-Reconfigurable Antenna. ACS Appl. Electron. Mater. 2023, 5, 1697-1706.
- Carter, C.; Kratish, Y.; Marks, T.J. Influence of Rare-Earth Ion Radius on Metal–Metal Charge Transfer in Trinuclear Mixed-Valent Complexes. Inorg. Chem. 2023, 62, 4799-4813.
- Wang, Y.; Zhang, Z.; Abergel, R.J. Hydroxypyridinone-based stabilization of Np(IV) enabling efficient U/Np/Pu separations in the Adapted PUREX process. Separation and Purification Technology 2021, 259, 118178.
- Lin, L.; Xu, N.; Wu, C.; Huang, J.; Nattestad, A.; Zheng, X.; Wallace, G.G.; Zhang, S.; Chen, J. Unzipping chemical bonds of non-layered bulk structures to form ultrathin nanocrystals. Matter 2021, 4, 955-968.
- Goes, S.L.; Mayer, M.N.; Nutting, J.E.; Hoober-Burkhardt, L.E.; Stahl, S.S.; Rafiee, M. Deriving the Turnover Frequency of Aminoxyl-Catalyzed Alcohol Oxidation by Chronoamperometry: An Introduction to Organic Electrocatalysis. J. Chem. Educ. 2021, 98, 600-606.
- Chaudhary, M.; Singh, M.; Kumar, A.; , .; Gautam, Y.K.; Malik, A.K.; Kumar, Y.; Singh, B.P. Experimental investigation of Co and Fe-Doped CuO nanostructured electrode material for remarkable electrochemical performance. Ceramics International 2021, 47, 2094-2106.
- Garcia, C.R.; Oliva, J.; Chávez, D.; Esquivel, B.; Gómez-Solís, C.; Martínez-Sánchez, E.; Mtz-Enriquez, A.I. Effect of Bismuth Dopant on the Photocatalytic Properties of SrTiO3 Under Solar Irradiation. Top Catal 2021, 64, 155-166.
- Chavez, D.; Gomez-Solis, C.; Mtz-Enriquez, A.I.; Rodriguez-Gonzalez, V.; Escobar-Barrios, V.; Garcia, C.R.; Oliva, J. High sensitivity of flexible graphene composites decorated with V2O5 microbelts for NO2 detection. Materials Research Bulletin 2021, 133, 111052.
- Saladin, M.; Maroncelli, M. Electron Transfer Kinetics between an Electron-Accepting Ionic Liquid and Coumarin Dyes. J. Phys. Chem. B 2020, 124, 11431-11445.
- Liu, M.; Lai, C.; Zhang, M.; Radu, D.R. Cascade synthesis and optoelectronic applications of intermediate bandgap Cu3VSe4 nanosheets. Sci. Rep. 2020, 10, 21679.
- Perez-Gonzalez, R.; Peng, Z.; Camacho, D.; Oliva, A.I.; Pei, Q.; Zakhidov, A.; Encinas, A.; Oliva, J. All solid state stretchable carbon nanotube based supercapacitors with controllable output voltage. Journal of Energy Storage 2020, 32, 101844.
- Lopez-Medina, M.; Hernandez-Navarro, F.; Mtz-Enriquez, A.I.; Oliva, A.I.; Rodriguez-Gonzalez, V.; Camarillo-Garcia, J.P.; Aguilar-Ortiz, C.O.; Flores-Zuñiga, H.; Oliva, J. Enhancing the capacity and discharge times of flexible graphene batteries by decorating their anodes with magnetic alloys NiMnMx (Mx=Ga, In, Sn). Materials Chemistry and Physics 2020, 256, 123660.
- Treviño, R.E.; Slater, J.W.; Shafaat, H.S. Robust Carbon-Based Electrodes for Hydrogen Evolution through Site-Selective Covalent Attachment of an Artificial Metalloenzyme. ACS Appl. Energy Mater. 2020, 3, 11099-11112.
- Chen, W.; Xie, C.; Wang, Y.; Zou, Y.; Dong, C.; Huang, Y.; Xiao, Z.; Wei, Z.; Du, S.; Chen, C.; Zhou, B.; Ma, J.; Wang, S. Activity Origins and Design Principles of Nickel-Based Catalysts for Nucleophile Electrooxidation. Chem 2020, 6, 2974-2993.
- Homayounfar, S.Z.; Rostaminia, S.; Kiaghadi, A.; Chen, X.; Alexander, E.T.; Ganesan, D.; Andrew, T.L. Multimodal Smart Eyewear for Longitudinal Eye Movement Tracking. Matter 2020, 3, 1275-1293.
- Wang, L.; Lin, Y.; DeCarlo, S.; Wang, Y.; Leung, K.; Qi, Y.; Xu, K.; Wang, C.; Eichhorn, B.W. Compositions and Formation Mechanisms of Solid-Electrolyte-Interphase (SEI) on Microporous Carbon/Sulfur Cathodes. Chem. Mater. 2020.
- Wijesinghe, M.S.; Batchelder, K.; Wickramasinghe, D.; Oh, J.; Chow, K. Battery-powered distance-based electrochemical sensor using a longitudinally-oriented silver band electrode. Sensors and Actuators B: Chemical 2020, 308, 127684.
- Nguyen, K.T.; Lane, E.E.; McMillen, C.D.; Pienkos, J.A.; Wagenknecht, P.S. Is Indenyl a Stronger or Weaker Electron Donor Ligand than Cyclopentadienyl? Opposing Effects of Indenyl Electron Density and Ring Slipping on Electrochemical Potentials. Organometallics 2020, 39, 670-678.
- Madrigal, E.A.; Taylor, J.K.; Raghu, G.; West, R.M. Cross-linking of DNA monolayers by cisplatin examined using electrostatic denaturation. Journal of Electroanalytical Chemistry 2020, 860, 113762.
- Jenks, T.C.; Kuda-Wedagedara, A.N.W.; Bailey, M.D.; Ward, C.L.; Allen, M.J. Spectroscopic and Electrochemical Trends in Divalent Lanthanides through Modulation of Coordination Environment. Inorg. Chem. 2020, 59, 2613-2620.
- Mtz-Enriquez, A.I.; Padmasree, K.P.; Oliva, A.I.; Gomez-Solis, C.; Coutino-Gonzalez, E.; Garcia, C.R.; Esparza, D.; Oliva, J. Tailoring the detection sensitivity of graphene based flexible smoke sensors by decorating with ceramic microparticles. Sensors and Actuators B: Chemical 2020, 305, 127466.
- Pallares, R.M.; Carter, K.P.; Zeltmann, S.E.; Tratnjek, T.; Minor, A.M.; Abergel, R.J. Selective Lanthanide Sensing with Gold Nanoparticles and Hydroxypyridinone Chelators. Inorg. Chem. 2020, 59, 2030-2036.
- Carpenter, J.M.; Zhong, F.; Ragusa, M.J.; Louro, R.O.; Hogan, D.A.; Pletneva, E.V. Structure and redox properties of the diheme electron carrier cytochrome c4 from Pseudomonas aeruginosa. Journal of Inorganic Biochemistry 2020, 203, 110889.
- McLoughlin, E.A.; Matson, B.D.; Sarangi, R.; Waymouth, R.M. Electrocatalytic Alcohol Oxidation with Iron-Based Acceptorless Alcohol Dehydrogenation Catalyst. Inorg. Chem. 2020, 59, 1453-1460.
- Wang, H.; Tampio, A.J.F.; Xu, Y.; Nicholas, B.D.; Ren, D. Noninvasive Control of Bacterial Biofilms by Wireless Electrostimulation. ACS Biomater. Sci. Eng. 2020, 6, 727-738.
- Hughes, M.A.; Allen, J.A.; Donne, S.W. Optimized Electrolytic Carbon and Electrolyte Systems for Electrochemical Capacitors. ChemElectroChem 2020, 7, 266-282.
- Liu, S.; Leng, J.; Aquino, T.C. Development of Disposable Single-Use Biosensor for Fructosyl Valine and Glycated Hemoglobin A1c. 2019, 09, 45.
- Müller, A.V.; de Oliveira, K.T.; Meyer, G.J.; Polo, A.S. Inhibiting Charge Recombination in cis-Ru(NCS)2 Diimine Sensitizers with Aromatic Substituents. ACS Appl. Mater. Interfaces 2019, 11, 43223-43234.
- Acharya, P.; Nelson, Z.J.; Benamara, M.; Manso, R.H.; Bakovic, S.I.P.; Abolhassani, M.; Lee, S.; Reinhart, B.; Chen, J.; Greenlee, L.F. Chemical Structure of Fe–Ni Nanoparticles for Efficient Oxygen Evolution Reaction Electrocatalysis. ACS Omega 2019, 4, 17209-17222.
- Kim, J.; Thomas, C.A.; Ewald, J.M.; Kurien, N.M.; Booker, M.E.; Greve, H.J.; Albu, T.V. Studies on lysozyme modifications induced by substituted p-benzoquinones. Bioorganic Chemistry 2019, 85, 386-398.
- Shao, J.; Johnson, A.; Hansen, C.A.; Kadish, K.M.; Han, B. Electroreductive dechlorination of γ-Hexachlorocyclohexane catalyzed by Rh2(dpf)4 in nonaqueous media, where dpf=N,N′-Diphenylformamidinate (1-) ion. Journal of Electroanalytical Chemistry 2019, 837, 208-218.
- Rahaman, A.; Ghosh, S.; Basak-Modi, S.; Abdel-Magied, A.F.; Kabir, S.E.; Haukka, M.; Richmond, M.G.; Lisensky, G.C.; Nordlander, E.; Hogarth, G. Chalcogenide-capped triiron clusters [Fe3(CO)9(μ3-E)2], [Fe3(CO)7(μ3-CO)(μ3-E)(μ-dppm)] and [Fe3(CO)7(μ3-E)2(μ-dppm)] (E = S, Se) as proton-reduction catalysts. Journal of Organometallic Chemistry 2019, 880, 213-222.
- King, A.P.; Gellineau, H.A.; MacMillan, S.N.; Wilson, J.J. Physical properties, ligand substitution reactions, and biological activity of Co(III)-Schiff base complexes. Dalton Trans. 2019.
- Ho, D. Detection and Melting of Surface-Bound DNA using a Purely Electrochemical Approach. Master's Thesis, University of San Francisco, 2018.
- Bindesri, S.D.; Alhatab, D.S.; Brosseau, C.L. Development of an electrochemical surface-enhanced Raman spectroscopy (EC-SERS) fabric-based plasmonic sensor for point-of-care diagnostics. Analyst 2018, 143, 4128-4135.
- Zhao, L.; Blackburn, J.; Brosseau, C.L. Quantitative Detection of Uric Acid by Electrochemical-Surface Enhanced Raman Spectroscopy Using a Multilayered Au/Ag Substrate. Anal. Chem. 2015, 87, 441-447.
- Harroun, S.G.; Abraham, T.J.; Prudhoe, C.; Zhang, Y.; Scammells, P.J.; Brosseau, C.L.; Pye, C.C.; Singer, R.D. Electrochemical surface-enhanced Raman spectroscopy (E-SERS) of novel biodegradable ionic liquids. Phys. Chem. Chem. Phys. 2013, 15, 19205-19212.
- Brown-Xu, S.E.; Chisholm, M.H.; Durr, C.B.; Spilker, T.F.; Young, P.J. Modulating the M2δ-to-ligand charge transfer transition by the use of diarylboron substituents. Dalton Trans. 2013, 42, 14491-14497.
- Budiyanto, E.; Ochoa-Hernández, C.; Tüysüz, H. Impact of Highly Concentrated Alkaline Treatment on Mesostructured Cobalt Oxide for the Oxygen Evolution Reaction. , 7, 2200499.
- Cicoira, F.; Wang, M.; Fan, J.; Calame, M.B.; Kim, C.; Chiang, C.; Segura, A.F.C.; Vurro, V.; Bargigia, I.; Mauzeroll, J. The “ins and outs” of electropolymerized PEDOT organic electrochemical transistors. .
- Piechota, E. Fundamental insights into electron transfer reactions of cyclometalated ruthenium donor-bridge-acceptor compounds. Ph.D. Dissertation, University of North Carolina at Chapel Hill, .
- Roth, H. INVESTIGATIONS TOWARD THE APPLICATION OF ORGANIC PHOTOREDOX CATALYSIS TO THE SYNTHESIS OF NATURAL PRODUCTS. Ph.D. Dissertation, University of North Carolina at Chapel Hill, .
- Brady, M. Fundamental Insights into Dye-Sensitized Interfaces for Solar Fuels Production. Ph.D. Dissertation, University of North Carolina at Chapel Hill, .
- McGuire, A. When a Hemoglobin Acts as a Catalytic Enzyme: Mechanistic Studies of Dehaloperoxidase. Ph.D. Dissertation, North Carolina State University, .
- Fleischmann, S.; Sun, Y.; Osti, N.C.; Wang, R.; Mamontov, E.; Jiang, D.; Augustyn, V. Interlayer separation in hydrogen titanates enables electrochemical proton intercalation. J. Mater. Chem. A , 8, 412-421.
- McLeod, K.E.R.; Lynk, T.P.; Sit, C.S.; Brosseau, C.L. On the origin of electrochemical surface-enhanced Raman spectroscopy (EC-SERS) signals for bacterial samples: the importance of filtered control studies in the development of new bacterial screening platforms - Analytical Methods (RSC Publishing). , 11, 924-929.
- Niepa, T.H.R.; Gilbert, J.L.; Ren, D. Controlling Pseudomonas aeruginosa persister cells by weak electrochemical currents and synergistic effects with tobramycin. Biomaterials , 33, 7356–7365.
- Pazdzior, R.; Yang, Z.(.; Mesbahuddin, M.S.; Yee, J.; van der Est, A.; Rafferty, S. Low reduction potential cytochrome b5 isotypes of Giardia intestinalis. Exp. Parasitol. , 157, 197-201.
- McDarmont, S.L.; McMillen, C.D.; Temelso, B.; Pienkos, J.A. Exploiting a C–F Activation Strategy to Generate Novel Tris(pyrazolyl)methane Ligands. , 646, 1886-1891.
- Wang, R.; Sun, Y.; Brady, A.; Fleischmann, S.; Eldred, T.B.; Gao, W.; Wang, H.; Jiang, D.; Augustyn, V. Fast Proton Insertion in Layered H2W2O7 via Selective Etching of an Aurivillius Phase. Adv. Energy Mater. , 11, 2003335.
- Jenks, T.C. The Effects of Coordination Environment on the Spectroscopic and Electrochemical Properties of Divalent Lanthanides. Ph.D. Dissertation, Wayne State University, .
- A. Hughes, M.; D. Bennett, R.; A. Allen, J.; W. Donne, S. Physical characteristics of capacitive carbons derived from the electrolytic reduction of alkali metal carbonate molten salts. RSC Adv. , 9, 36771-36787.
- Oh, S.; Park, H.; Kim, H.; Park, Y.S.; Ha, M.G.; Jang, J.H.; Kim, S. Fabrication of Large Area Ag Gas Diffusion Electrode via Electrodeposition for Electrochemical CO2 Reduction. Coatings , 10, 341.
- Neshani, S.; Nyamekye, C.K.A.; Melvin, S.; Smith, E.A.; Chen, D.J.; Neihart, N.M. AC and DC Differential Bridge Structure Suitable for Electrochemical Interfacial Capacitance Biosensing Applications. Biosensors , 10, 28.
- Dupont, M.F.; Gibson, A.J.; Elbourne, A.; Forghani, M.; Cross, A.D.; Donne, S.W. In Situ Investigation of the Electrodeposition Mechanism of Manganese Dioxide from a Citrate Electrolyte: The Effect of Intermediate Stabilization on Material Morphology. J. Electrochem. Soc. , 167, 040520.
- Balaraman, L.; Emhoff, K.A.; Salem, A.M.; Hanna, J.; Alsabony, M.N.; Bayachou, M.; Mundell, J.J.; Christopher Boyd, W. Electrochemical studies of cobalt(II) diphenylazodioxide complexes. Inorganica Chimica Acta , 501, 119277.
- Ouellette, M.; Mathault, J.; Niyonambaza, S.D.; Miled, A.; Boisselier, E. Electrochemical Detection of Dopamine Based on Functionalized Electrodes. Coatings , 9, 496.
- Kellett, C.W.; Swords, W.B.; Turlington, M.D.; Meyer, G.J.; Berlinguette, C.P. Resolving orbital pathways for intermolecular electron transfer. Nat. Commun. , 9, 4916.
- Mughal, M.A.; Alqudsi, A.; Rao, P.M.; Masroor, M.; Ichwani, R.; Zhou, L.; Giri, B. All-electrodeposited p-Cu2ZnSnS4/n-In2S3 Heterojunction Formation for Solar Cell Applications. , 142–147.
- Brown, T.A.; Chen, H.; Zare, R.N. Detection of the Short-Lived Radical Cation Intermediate in the Electrooxidation of N,N -Dimethylaniline by Mass Spectrometry. , 127, 11335–11337.
- Singh, P.; Saltsman, I.; Mahammed, A.; Goldberg, I.; Tumanskii, B.; Gross, Z. Iron complexes of tris(4-nitrophenyl)corrole, with emphasis on the (nitrosyl)iron complex. J. Porphyrins Phthalocyanines , 16, 663–673.
- Mahammed, A.; Tumanskii, B.; Gross, Z. Effect of bromination on the electrochemistry, frontier orbitals, and spectroscopy of metallocorroles. J. Porphyrins Phthalocyanines , 15, 1275–1286.
- Baker, D.; Davis, S.; Budner, D. Electrochemical Detection of Glucose Using a Prussian Blue Modified Electrodes Combined with Glucose Oxidase Encapsulated within a Xerogel. Journal of the South Carolina Academy of Science , 22, 6-9.
Related Products and Accessories
-

E1P Series Stationary Working Electrode, Glassy Carbon (GC), 85 mm length, PTFE shroud
AFE1PGC -

Reference Electrode, Silver/Silver Chloride, LowProfile, 74 mm Long
RRPEAGCL -

Three Electrode Cell Kit, Low Volume
AF01CKT1006 -

Screen-Printed Electrode Cell Kit
AKSPEKIT -
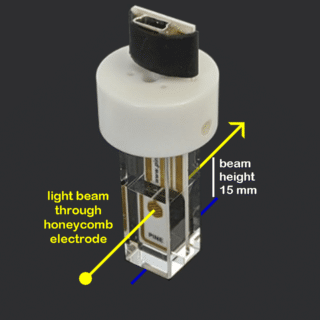
Honeycomb Spectroelectrochemistry Cell Kit
AKSTCKIT3 -
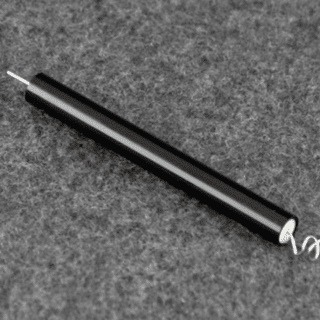
Counter Electrode, Platinum (Pt), LowProfile
RRPG257PT









