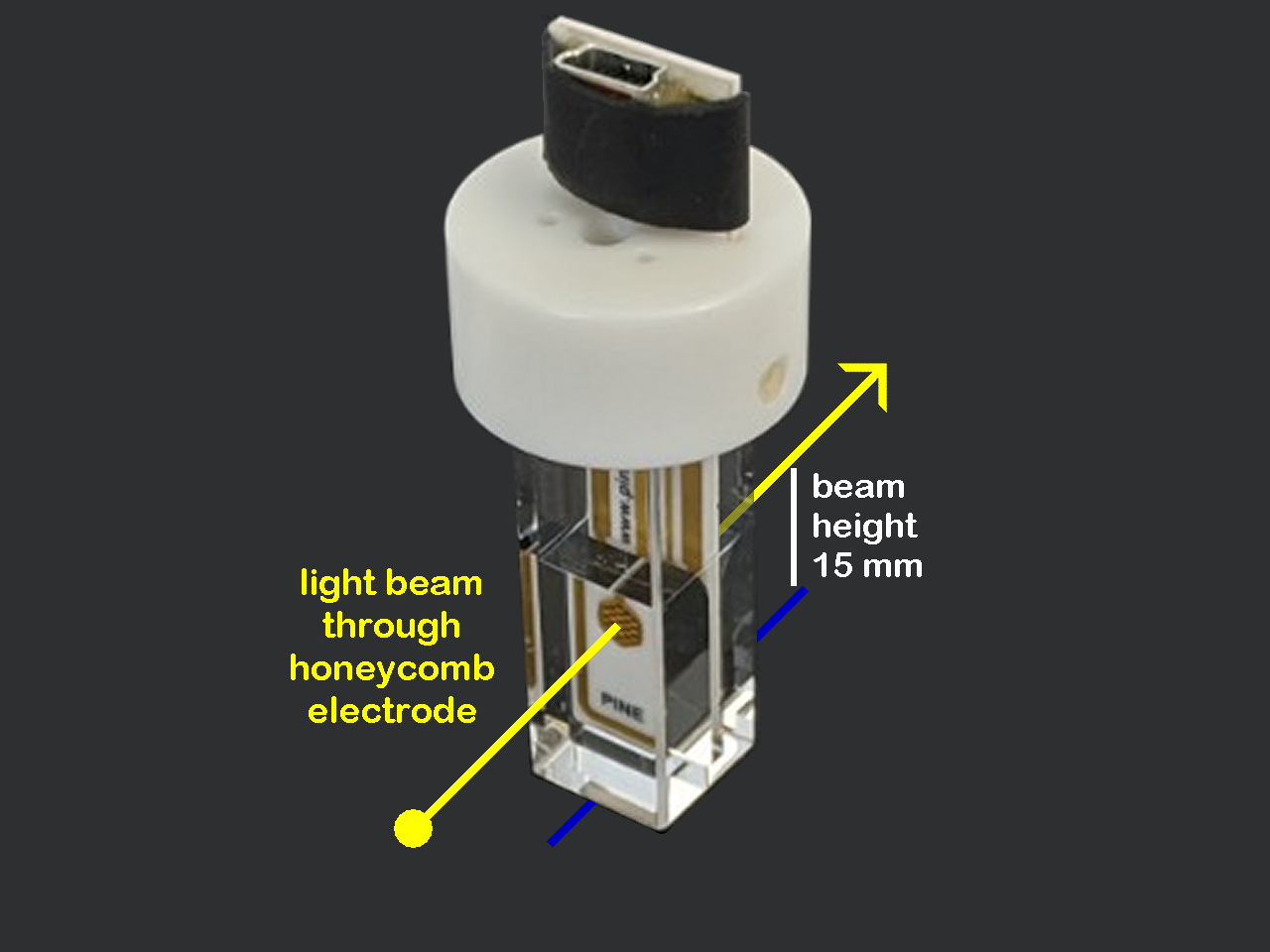
Honeycomb Spectroelectrochemistry Cell Kit
Our unique UV/Vis spectroelectrochemical cell features a patterned “honeycomb” electrode which mounts easily inside a thin-layer quartz cuvette. A special cuvette cap securely holds the honeycomb electrode card and a separate reference electrode in the proper position within the cuvette.
This product is sold as a kit, meaning it consists of multiple independent products used together. View the individual kit products in the tab below.
This product is available in related bundles. View related bundles in the tab below.
Customers must be logged into their account to view prices. Not all regions provide pricing online. If you do not see prices, you can obtain them from the designated sales channel in your region.
Complete SpectroelectrochemistryCell Kit: The Honeycomb Spectroelectrochemical Cell Kit (AKSTCKIT3) includes 1 quartz cuvette, 1 cell cap, 1 platinum honeycomb electrode, 2 gold honeycomb electrodes, the Universal Specialty Cell Connection Kit, and a LowProfile Ag/AgCl reference electrode. Our honeycomb electrode chip contains an onboard working, counter and reference electrode. If an alternative reference electrode is needed, we offer a cell cable with a separate reference breakout lead with each Honeycomb cell, for use with any potentiostat.
- , . In-situ spectroelectrochemical analysis: Irreversible deformation of cesium lead bromide Perovskite Quantum Dots in SiOx matrices. 2024, 8, 100208.
- Sahil, S.T.; McCardle, K.M.; Le Magueres, P.; Panetier, J.A.; Jurss, J.W. Investigations of a Copper(II) Bipyridyl-N-Heterocyclic Carbene Macrocycle for CO2 Reduction: Apparent Formation of an Imidazolium Carboxylate Intermediate Leading to Demetalation. ACS Omega 2024, 9, 34555-34566.
- Milunovic, M.N.M.; Ohui, K.; Besleaga, I.; Petrasheuskaya, T.V.; Dömötör, O.; Enyedy, É.A.; Darvasiova, D.; Rapta, P.; Barbieriková, Z.; Vegh, D.; Tóth, S.; Tóth, J.; Kucsma, N.; Szakács, G.; Popović-Bijelić, A.; Zafar, A.; Reynisson, J.; Shutalev, A.D.; Bai, R.; Hamel, E.; Arion, V.B. Copper(II) Complexes with Isomeric Morpholine-Substituted 2-Formylpyridine Thiosemicarbazone Hybrids as Potential Anticancer Drugs Inhibiting Both Ribonucleotide Reductase and Tubulin Polymerization: The Morpholine Position Matters. J. Med. Chem. 2024, 67, 9069-9090.
- Kovács, H.; Jakusch, T.; May, N.V.; Tóth, S.; Szakács, G.; Enyedy, É.A. Complex formation of ML324, the histone demethylase inhibitor, with essential metal ions: Relationship between solution chemistry and anticancer activity. Journal of Inorganic Biochemistry 2024, 255, 112540.
- Gemünde, A.; Gail, J.; Holtmann, D. Redox mediator interaction with Cupriavidus necator – spectroelectrochemical online analysis. Electrochemistry Communications 2024, 162, 107705.
- Portela, P.C.; Shipps, C.C.; Shen, C.; Srikanth, V.; Salgueiro, C.A.; Malvankar, N.S. Widespread extracellular electron transfer pathways for charging microbial cytochrome OmcS nanowires via periplasmic cytochromes PpcABCDE. Nat Commun 2024, 15, 2434.
- Carpenter, J.M.; Zhong, F.; Ragusa, M.J.; Louro, R.O.; Hogan, D.A.; Pletneva, E.V. Structure and redox properties of the diheme electron carrier cytochrome c4 from Pseudomonas aeruginosa. Journal of Inorganic Biochemistry 2020, 203, 110889.
- Darvasiová, D.; Šoral, M.; Puškárová, I.; Dvoranová, D.; Vénosová, B.; Bučinský, L.; Zalibera, M.; Dujnič, V.; Dobrov, A.; Schwalbe, M.; Arion, V.B.; Rapta, P. Spectroelectrochemical, photochemical and theoretical study of octaazamacrocyclic nickel(II) complexes exhibiting unusual solvent-dependent deprotonation of methylene group. Electrochimica Acta 2019, 326, 135006.
- Zheng, W.; Tsang, C.; So, L.Y.; Liu, M.; Leung, Y.; Lee, L.Y.S. Highly efficient stepwise electrochemical degradation of antibiotics in water by in situ formed Cu(OH)2 nanowires. Applied Catalysis B: Environmental 2019, 256, 117824.
- Dobrov, A.; Darvasiová, D.; Zalibera, M.; Bučinský, L.; Puškárová, I.; Rapta, P.; Shova, S.; Dumitrescu, D.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Arion, V.B. Nickel(II) Complexes with Redox Noninnocent Octaazamacrocycles as Catalysts in Oxidation Reactions. Inorg. Chem. 2019, 58, 11133-11145.
- Ohui, K.; Babak, M.V.; Darvasiova, D.; Roller, A.; Vegh, D.; Rapta, P.; Guan, G.R.S.; Ou, Y.H.; Pastorin, G.; Arion, V.B. Redox-Active Organoruthenium(II)– and Organoosmium(II)–Copper(II) Complexes, with an Amidrazone–Morpholine Hybrid and [CuICl2]− as Counteranion and Their Antiproliferative Activity. Organometallics 2019, 38, 2307-2318.
- Ohui, K.; Afanasenko, E.; Bacher, F.; Ting, R.L.X.; Zafar, A.; Blanco-Cabra, N.; Torrents, E.; Dömötör, O.; May, N.V.; Darvasiova, D.; Enyedy, É.A.; Popović-Bijelić, A.; Reynisson, J.; Rapta, P.; Babak, M.V.; Pastorin, G.; Arion, V.B. New Water-Soluble Copper(II) Complexes with Morpholine–Thiosemicarbazone Hybrids: Insights into the Anticancer and Antibacterial Mode of Action. J. Med. Chem. 2019, 62, 512-530.
- Schorsch, M.; Kramer, M.; Goss, T.; Eisenhut, M.; Robinson, N.; Osman, D.; Wilde, A.; Sadaf, S.; Brückler, H.; Walder, L.; Scheibe, R.; Hase, T.; Hanke, G.T. A unique ferredoxin acts as a player in the low-iron response of photosynthetic organisms. PNAS 2018, 115, E12111-E12120.
- Orlowska, E.; Babak, M.V.; Dömötör, O.; Enyedy, E.A.; Rapta, P.; Zalibera, M.; Bučinský, L.; Malček, M.; Govind, C.; Karunakaran, V.; Farid, Y.C.S.; McDonnell, T.E.; Luneau, D.; Schaniel, D.; Ang, W.H.; Arion, V.B. NO Releasing and Anticancer Properties of Octahedral Ruthenium–Nitrosyl Complexes with Equatorial 1H-Indazole Ligands. Inorg. Chem. 2018, 57, 10702-10717.
- Piechota, E.J.; Troian-Gautier, L.; Sampaio, R.N.; Brennaman, M.K.; Hu, K.; Berlinguette, C.P.; Meyer, G.J. Optical Intramolecular Electron Transfer in Opposite Directions through the Same Bridge That Follows Different Pathways. J. Am. Chem. Soc. 2018, 140, 7176-7186.
- Barr, T.J.; Morris, A.J.; Taheri, A.; Meyer, G.J. Charge Rectification at Molecular Nanocrystalline TiO2 Interfaces: Overlap Optimization To Promote Vectorial Electron Transfer. J. Phys. Chem. C 2016, 120, 27173-27181.
- Liang, Z.; Kang, M.; Payne, G.F.; Wang, X.; Sun, R. Probing Energy and Electron Transfer Mechanisms in Fluorescence Quenching of Biomass Carbon Quantum Dots. ACS Appl. Mater. Interfaces 2016, 8, 17478-17488.
- Salpage, S.R.; Paul, A.; Som, B.; Banerjee, T.; Hanson, K.; Smith, M.D.; Vannucci, A.K.; Shimizu, L.S. Structural, electrochemical and photophysical properties of an exocyclic di-ruthenium complex and its application as a photosensitizer. Dalton Trans. 2016, 45, 9601-9607.
- Kim, J.; Yennawar, H.P.; Lear, B.J. Synthesis and characterization of ruthenium polypyridyl complexes with hydroxypyridine derivatives: effect of protonation and ethylation at the pyridyl nitrogen. Dalton Trans. 2013, 42, 15656-15662.
- Thomsen, J.M.; Sheehan, S.W.; Hashmi, S.M.; Campos, J.; Hintermair, U.; Crabtree, R.H.; Brudvig, G.W. Electrochemical Activation of Cp* Iridium Complexes for Electrode-Driven Water-Oxidation Catalysis. J. Am. Chem. Soc. , 136, 13826–13834.
- Zhong, F.; Lisi, G.P.; Collins, D.P.; Dawson, J.H.; Pletneva, E.V. Redox-dependent stability, protonation, and reactivity of cysteine-bound heme proteins. , 111, E306—-E315.
- Kennedy, S.R.; Kozar, M.N.; Yennawar, H.P.; Lear, B.J. Synthesis and characterization of the gold dithiolene monoanion, (Bu4N)[Au(pdt=2,3-pyrazinedithiol)2]. Polyhedron , 103, 100–104.
- Pourrieux, G.; Abate, P.O.; Vergara, M.M.; Katz, N.E. Redox-induced linkage isomerization detected in [Ru(NH3)5(NVF)](PF6)2(NVF=N-vinylformamide). Inorg. Chem. Commun. , 66, 90-93.
- Yang, Z.(.; Pazdzior, R.; Yee, J.; Rafferty, S. Reduction potential and heme-pocket polarity in low potential cytochrome b5 of Giardia intestinalis. J. Inorg. Biochem. , 158, 110–114.
- Shova, S.; Vlad, A.; Cazacu, M.; Krzystek, J.; Bucinsky, L.; Breza, M.; Darvasiová, D.; Rapta, P.; Cano, J.; Telser, J.; B. Arion, V. A five-coordinate manganese(III) complex of a salen type ligand with a positive axial anisotropy parameter D. Dalton Trans. , 46, 11817-11829.
- Shova, S.; Vlad, A.; Cazacu, M.; Krzystek, J.; Ozarowski, A.; Malček, M.; Bucinsky, L.; Rapta, P.; Cano, J.; Telser, J.; B. Arion, V. Dinuclear manganese(III) complexes with bioinspired coordination and variable linkers showing weak exchange effects: a synthetic, structural, spectroscopic and computation study. Dalton Trans. .
- E. Büchel, G.; Kossatz, S.; Sadique, A.; Rapta, P.; Zalibera, M.; Bucinsky, L.; Komorovsky, S.; Telser, J.; Eppinger, J.; Reiner, T.; B. Arion, V. cis -Tetrachlorido-bis(indazole)osmium(IV) and its osmium(III) analogues: paving the way towards the cis -isomer of the ruthenium anticancer drugs KP1019 and/or NKP1339. Dalton Trans. , 46, 11925-11941.
- Brady, M. Fundamental Insights into Dye-Sensitized Interfaces for Solar Fuels Production. Ph.D. Dissertation, University of North Carolina at Chapel Hill, .
- Pazdzior, R.; Yang, Z.(.; Mesbahuddin, M.S.; Yee, J.; van der Est, A.; Rafferty, S. Low reduction potential cytochrome b5 isotypes of Giardia intestinalis. Exp. Parasitol. , 157, 197-201.
- Al-Yasari, A. Synthesis, non-linear optical and electrochemical properties of novel organoimido polyoxometalate derivatives. Ph.D. Dissertation, University of East Anglia, .
- Balaraman, L.; Emhoff, K.A.; Salem, A.M.; Hanna, J.; Alsabony, M.N.; Bayachou, M.; Mundell, J.J.; Christopher Boyd, W. Electrochemical studies of cobalt(II) diphenylazodioxide complexes. Inorganica Chimica Acta , 501, 119277.
- Kellett, C.W.; Swords, W.B.; Turlington, M.D.; Meyer, G.J.; Berlinguette, C.P. Resolving orbital pathways for intermolecular electron transfer. Nat. Commun. , 9, 4916.
- Adams, R.E.; Schmehl, R.H. Micellar Effects on Photoinduced Electron Transfer in Aqueous Solutions Revisited: Dramatic Enhancement of Cage Escape Yields in Surfactant Ru(II) Diimine Complex/[Ru(NH3)6]2+ Systems. Langmuir , 32, 8598–8607.
- DiMarco, B.N.; O'Donnell, R.M.; Meyer, G.J. Cation-Dependent Charge Recombination to Organic Mediators in Dye-Sensitized Solar Cells. J. Phys. Chem. C , 119, 21599–21604.
- Zhao, Y.; Vargas-Barbosa, N.M.; Strayer, M.E.; McCool, N.S.; Pandelia, M.; Saunders, T.P.; Swierk, J.R.; Callejas, J.F.; Jensen, L.; Mallouk, T.E. Understanding the Effect of Monomeric Iridium(III/IV) Aquo Complexes on the Photoelectrochemistry of IrOx·nH2O-Catalyzed Water-Splitting Systems. J. Am. Chem. Soc. , 137, 8749–8757.
- Bischof, A.M.; Zhang, S.; Meyer, T.Y.; Lear, B.J. Quantitative Assessment of the Connection between Steric Hindrance and Electronic Coupling in 2,5-Bis(alkoxy)benzene-Based Mixed-Valence Dimers. J. Phys. Chem. C , 118, 12693–12699.
- Navarathne, D.; Skene, W.G. Towards Electrochromic Devices Having Visible Color Switching Using Electronic Push–Push and Push–Pull Cinnamaldehyde Derivatives. ACS Appl. Mater. Interfaces , 5, 12646–12653.
- King, J.D.; McIntosh, C.L.; Halsey, C.M.; Lada, B.M.; Niedzwiedzki, D.M.; Cooley, J.W.; Blankenship, R.E. Metalloproteins Diversified: The Auracyanins Are a Family of Cupredoxins That Stretch the Spectral and Redox Limits of Blue Copper Proteins. Biochemistry (Mosc.) , 52, 8267–8275.
- Palmer, J.H.; Lancaster, K.M. Molecular Redox: Revisiting the Electronic Structures of the Group 9 Metallocorroles. Inorg. Chem. , 51, 12473–12482.
- Lee, C.; Kim, K.; Shin, Y.; Han, D.; Yoon, S.J. In Situ Spectroelectrochemical Investigation of Perovskite Quantum Dots for Tracking Their Transformation. Front. Energy Res. , 8, -.
- Zhang, D.; E. Rosch, L.; R. Crawley, M.; R. Cook, T. Post-synthetic modification of bis-iron( iii )-μ-oxo-porphyrin prisms to enhance oxygen reduction electrocatalysis. Inorg. Chem. Front. , 11, 5557-5565.