Low Volume Cell Series Cap Kit
Part Number
AF01CKT1004
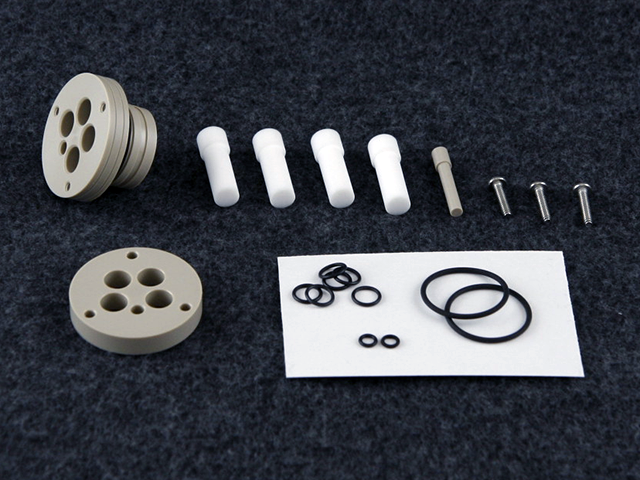
Pine Research’s Low Volume Cap Kit can be used in two configurations – either open or closed to the atmosphere. The cap kit has the option for sealing around all electrodes and probes in the cell with optimally-sized fluoroelastomer O-rings and a sealing mechanical cap. Fluoroelastomer (FKM) O-rings are not chemically compatible in all solvent systems. FKM provides good coverage of many commonly used solvents. Customers using more aggressive solvents are encouraged to source their own O-rings to suit their application. This cap kit is included with each Low Volume Cell Kit. Replacements can be purchased as a kit. The cap is machined from chemically-resistant PEEK polymer.
Pine Research's Low Volume Cap Kit can be used in two configurations - either open or closed to the atmosphere. The cap kit has the option for sealing around all electrodes and probes in the cell with optimally-sized fluoroelastomer O-rings and a sealing mechanical cap. Fluoroelastomer (FKM) O-rings are not chemically compatible in all solvent systems. FKM provides good coverage of many commonly used solvents. Customers using more aggressive solvents are encouraged to source their own O-rings to suit their application. This cap kit is included with each Low Volume Cell Kit. Replacements can be purchased as a kit. The cap is machined from chemically-resistant PEEK polymer.
When possible, we add published articles, theses and dissertations, and books to our references library. When we know this product has been used, we will include it in this list below. If you have a reference where our product was used and it's not in this list, please contact us with the details and we will add it.
- Bard et al. Electrochemical Methods: Fundamentals and Applications, 3rd ed; John Wiley & Sons, Ltd: Hoboken, NJ, 2022.
- Orazem and Tribollet Electrochemical impedance spectroscopy, 2nd ed; John Wiley & Sons, Inc.: Hoboken, NJ, 2017.
- Leddy, J., Paschkewitz, T. Ammonia production using bioelectrocatalytical devices. US9506085B2, 2016.
- Esrafilzadeh et al. Room temperature CO2 reduction to solid carbon species on liquid metals featuring atomically thin ceria interfaces. Nature Communications, 2019, 10, 865.
- Ashok et al. Single Step Synthesis of Porous NiCoO2 for Effective Electrooxidation of Glycerol in Alkaline Medium. Journal of The Electrochemical Society, 2018, 165, J3301-J3309.
- Yang and Venton. Carbon Nanomaterials for Neuroanalytical Chemistry. In Nanocarbons for Electroanalysis; Szunerits, S., Boukherroub, R., Downard, A., Zhu, J. Eds; John Wiley & Sons, Ltd: Chichester, UK, 2017-09; pp 55-83.
- Baker et al. Organophosphate vapor detection on gold electrodes using peptide nanotubes. Biosensors and Bioelectronics, 2014, 61, 119–123.
- Paschkewitz, Timothy. Ammonia Production at Ambient Temperature and Pressure. Ph.D. Dissertation, University of Iowa (Iowa City, Iowa), 2012-01.
Visit our YouTube channel for helpful instructional videos regarding the use and maintenance of this and many other Pine Research products!